Tissue Biomechanics
Physicians have been performing manual palpation on patients for millennia since diseases can alter tissue biomechanical properties. However, quantitative mechanical imaging was only formalized in the early 1990s with magnetic resonance elastography (MRE) and ultrasound elastography (USE). Our lab specializes in the development and applications of optical coherence elastography (OCE), which is an optical alternative to MRE and USE. OCE has higher resolution, enabling noninvasive mechanical imaging at the micrometer-scale. OCE is also highly sensitive to motion (nm-scale displacement sensitivity), enabling the use of minimal forces for safe tissue stimulations and minimizing the effect of global motion and non-linear effects.
Optical Coherence Elastography
Wave-based OCE
One of the most common OCE methods relies on quantifying the properties of elastic waves in tissues. Mechanical properties such as Young’s modulus and shear viscosity can be estimated from the speed, dispersion, damping, and other properties of the elastic wave. The Biomedical Optics Lab has successfully utilized wave-based OCE to study the biomechanical properties of numerous tissues, such as the cornea, iris, limbus, sclera, skin, colon, kidneys, cardiovascular tissues, and connective tissues. These waves can be excited by a variety of methods tailored to the specific application. For example, mechanical tapping, pneumatic methods, or acoustic radiation force stimulation can all excite mechanical waves in tissues noninvasively.
 Mechanical wave propagation in a (left) untreated and (right) cross-linked ex vivo porcine cornea. Singh et al., IEEE JSTQE, 2016.
Mechanical wave propagation in a (left) untreated and (right) cross-linked ex vivo porcine cornea. Singh et al., IEEE JSTQE, 2016.
Nanobomb OCE
The Biomedical Optics Lab has developed an exotic method for exciting highly localized and targeted mechanical waves in collaboration with Biomedical Optics and NanoDiagnostics (BOND) laboratory at the University of Texas MD Anderson Cancer Center. Perfluorocarbon (PFC) is mixed with an optically absorbing dye in lipid nanodroplets, called nanobombs. A pulsed laser causes the PFC to rapidly vaporize, which causes near instantaneous expansion of the nanobombs, inducing highly localized mechanical vibrations that propagate as mechanical waves. These waves are very low amplitude (< 10 µm), which can be detected by OCE.
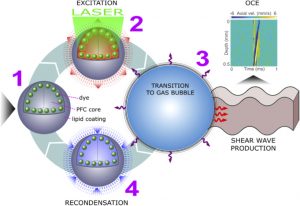
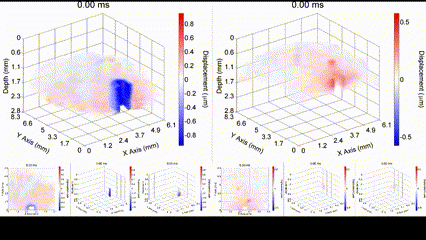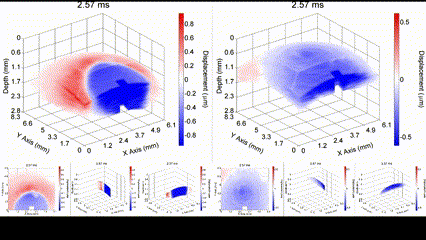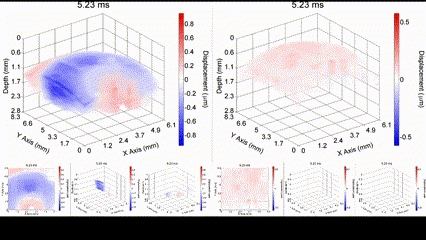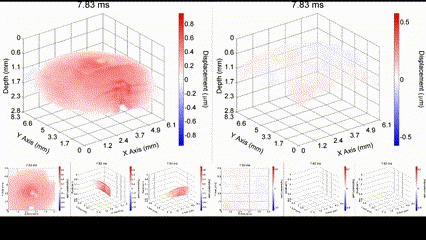
Illustration of a PFC nanobomb and generation of elastic waves. (1) A nanobomb consists of the core coated with a lipid shell with embedded dye molecules. (2) Pulsed laser energy is absorbed by the dye molecules that heat the core leading to (3) a rapid transient liquid-to-gas transition and expansion. The expansion generates a mechanical wave that is detected by OCE. (4) The bubble spontaneously recondenses within a few milliseconds back to the initial droplet (1) and the cycle can be repeated again. Boerner et al., Biomed Opt Express, 2020.
Compression OCE
In contrast to dynamic OCE, quasi-static OCE can perform more rapid and higher-resolution imaging where the micro-scale displacement between an uncompressed and compressed image is quantified. The Biomedical Optics lab has recently begun utilizing compression OCE for assessing the stiffness of the various tissues such as the cornea.
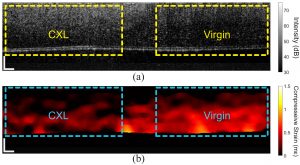
In vivo compression OCE of a rabbit cornea where the left half was crosslinked (CXL) and the right half was untreated. (a) OCT structural image and (b) strain map. Singh et al., Photonics, 2021.
Heartbeat OCE
An interesting new approach for OCE is to utilize no external stimulation, but rather, rely on the displacements induced by physiological processes. For example, the heartbeat-induced ocular pulse causes minute fluctuations in the cornea, which can be detected by OCE. This method enables measurements of corneal biomechanical properties with no active stimulation, which is beneficial because existing OCT instruments widely available in the clinic can be utilized for measuring tissue biomechanical properties.
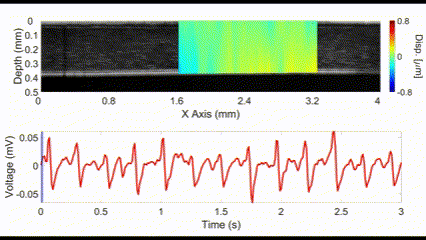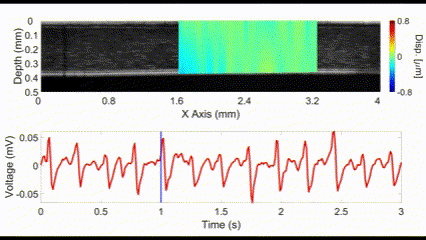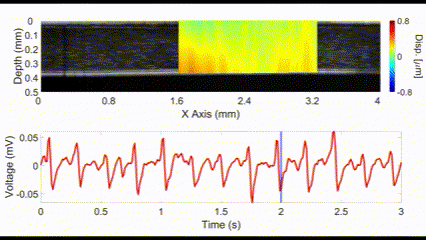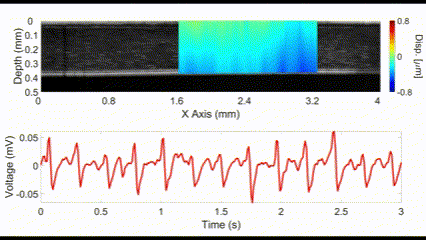
(top) OCE-measured displacement mapped on the OCT structural image of a rabbit cornea in vivo. (bottom) The heartbeat of the rabbit corresponding to the OCE-measured displacement. Nair et al., J Biomed Opt, 2021.
Reverberant OCE
Traditional elastography based on transversely propagating mechanical waves has limited mechanical resolution and contrast. These limitations can be overcome with reverberant elastography. Here, multiple mechanical waves are excited, which then interfere in the sample, forming a reverberant shear field. The analytical model of the reverberant shear field is then fitted to the 2D local autocorrelation of the reverberant shear field, giving the local elastic wave speed. Previous work has utilized contact-based excitation methods, which are not conducive for imaging small and delicate tissues, such as small animal embryos. Hence, the Biomedical Optics Laboratory has developed a novel noncontact method of exciting a reverberant shear field with a multi-focus acoustic lens.
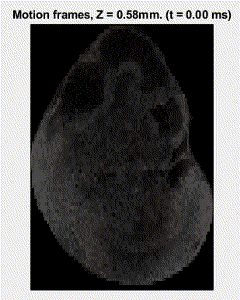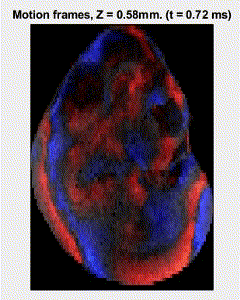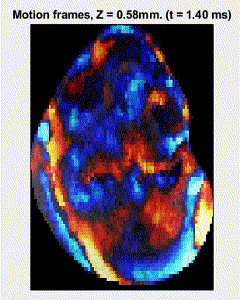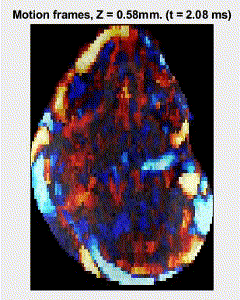
OCE imaging of a reverberant shear field in a murine embryo.
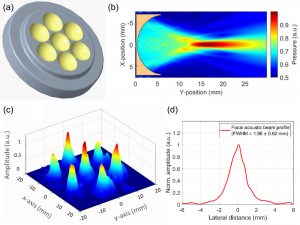
Multi-focus acoustic lens for noncontact reverberant OCE. (a) Design of the multi-focus lens. (b) Acoustic pressure field of a single element. (c) Acoustic pressure distribution at the focal plane. (d) Average acoustic pressure profile of all 7 spots. Mekonnen et al., Opt Lett, 2023.
Multimodal optical elastography
OCE relies on backscattered light, and thus, is a powerful technique for quantifying the biomechanical properties of scattering tissues. However, this limits its applicability in optically transparent tissues like the crystalline lens. To overcome this limitation, we have combined OCE with Brillouin microscopy. Brillouin microscopy is a non-invasive high-resolution all-optical imaging modality capable of mapping tissue stiffness, which is optimized for transparent tissues but cannot provide quantitative biomechanical parameters. By combining both methods, we can map quantitative biomechanical properties, such as Young’s modulus, of transparent tissues with high resolution.
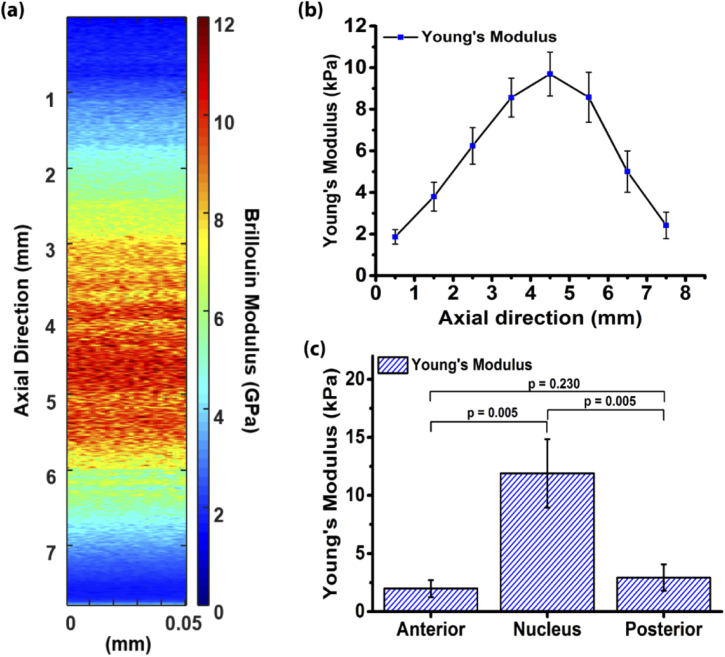
Quantitative multimodal optical elastography of the crystalline lens with OCE and Brillouin microscopy. (a) Axial map of the Brillouin (also known as longitudinal modulus) and (b) Young’s modulus of a crystalline lens. (c) Comparison of the elasticity of the different lens regions. Ambekar et al., Biomed Opt Express, 2020.
Developmental Biology
Another major area of research of the Biomedical Optics Laboratory is structural and functional imaging of embryonic development. The healthy development of embryos depends on several complex and critical processes, such as neurulation and the formation of the cardiovascular system. Small animal models are commonly used in developmental biology, and common medical imaging modalities lack the resolution and contrast for imaging the micro-scale tissues in these animal models. Thus, the Biomedical Optics Laboratory is focused on the development and application of various high-resolution and noninvasive imaging modalities to image small animal embryos. The techniques used in the laboratory include optical coherence tomography (OCT), optical projection tomography (OPT), and selective plane illumination microscopy (SPIM) otherwise known as lightsheet microscopy.
Optical Coherence Tomography
Optical coherence tomography (OCT) has emerged as a preferred method for small animal embryo imaging due to its noninvasive nature, high-resolution, and functional extensions. The Biomedical Optics Laboratory is developing new OCT techniques and applications for investigating biological questions in developmental biology, such as quantifying the effects of teratogens on organogenesis and brain vasculature.

3D OCT image of a zebrafish embryo.
Functional OCT
Embryo cardiodynamics
The vertebrate heart is the first functional organ that develops. The heart rapidly goes through many morphological, functional, and mechanical changes in order to properly pump blood throughout the developing embryo. Failure to properly form the heart and rest of the cardiovascular system can lead to significant cardiac congenital defects, which are the most common congenital defects. Thus, it is crucial to understand the mechanisms that regulate and drive cardiac development. OCT is perfectly suited for investigating cardiac development in small animal models of cardiac developmental diseases due to its micrometer spatial resolution, video-rate imaging, excellent contrast, and depth penetration of 1 to 2 mm in tissue.

4D OCT imaging of an E9.5 embryo heart. Wang et al., Opt Lett, 2015.
OCT angiography
Beyond structural imaging, OCT also has angiographic capabilities. The Biomedical Optics Laboratory has developed in utero imaging protocols for assessing the brain vasculature of murine embryos. This imaging technique has been utilized to assess the effects of various teratogens, such as alcohol, nicotine, and cannabinoids, on the vasculature of murine embryos at various development stages.
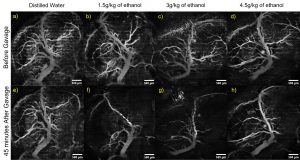
In utero OCT angiography of murine embryos. The mother was dosed with the noted liquids via intragastric gavage, and OCT angiography imaging was performed before and 45 minutes after gavage. Raghunathan et al., J Biomed Opt, 2020.
OPT imaging
OPT is an optical analog to X-ray computed tomography but uses fluorescence instead of ionizing radiation for imaging. A sample is cleared, stained, and mounted for OPT imaging, which provides beautiful high-contrast and high-resolution whole sample imaging.
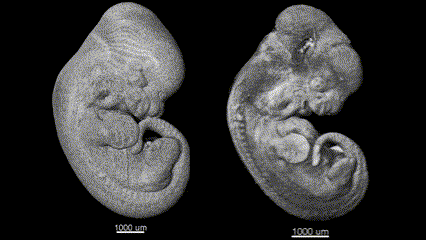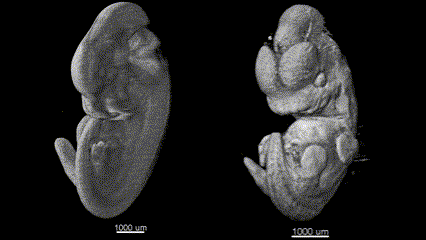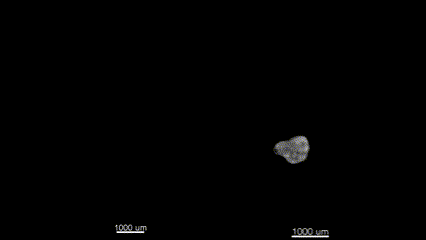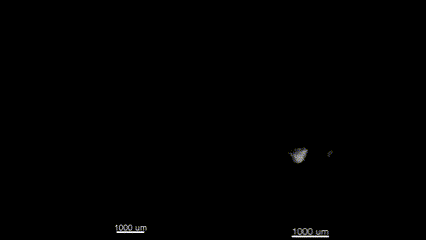
3D (left) OCT and (right) OPT images of the same E11.5 mouse embryo. Singh et al., Biomed Opt Express, 2016.
Multimodal Imaging
All imaging modalities have drawbacks, such as resolution and depth penetration or structural and functional specificity. To overcome these limitations, the Biomedical Optics Laboratory is developing novel multimodal optical imaging techniques for investigating various biological questions.
OCT & SPIM
Although OCT can provide micrometer-scale resolution images, it lacks molecular specificity. Thus, we have combined OCT with SPIM to combine structural imaging and functional fluorescence imaging. By co-aligning the systems, co-registration of the images acquired by both system is trivial.
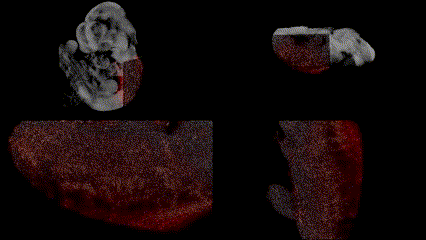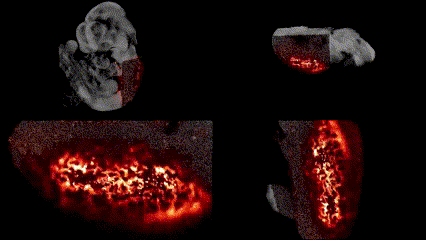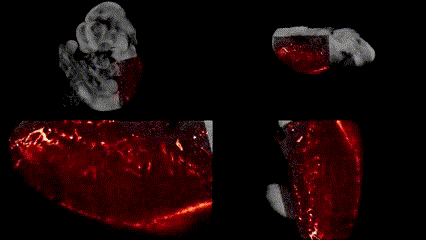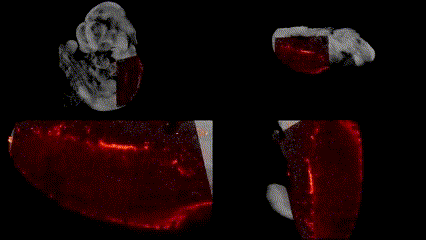
OCT structural imaging and SPIM imaging of GFP-labeled erythroblasts. Khajavi et al., Opt Lett, 2021.
OCT & Brillouin microscopy
During embryogenesis, there are many complex processes that are required for healthy development. However, these processes can be disturbed, leading to developmental defects. One such process is the formation of the neural tube, called neurulation. It is postulated that the interaction between tissue stiffness and mechanical forces regulate proper neurulation. Thus, it is important to understand the interplay between forces and tissue stiffness during development. Due to the sub-optimal measurement techniques, this relationship remains poorly understood. Brillouin microscopy is a non-invasive high-resolution all-optical imaging modality capable of mapping tissue stiffness. However, it lacks the ability to provide structural images and, thus, is severely limited when imaging dynamic processes such as neurulation. To overcome this limitation, we have combined Brillouin microscopy with OCT in one synchronized and co-aligned instrument. We developed a custom instrumentation software that utilizes the OCT structural image to guide Brillouin imaging. With this multimodal Brillouin+OCT system, we are investigating the effects of various genetic abnormalities on neural tube morphology and stiffness during neurulation.
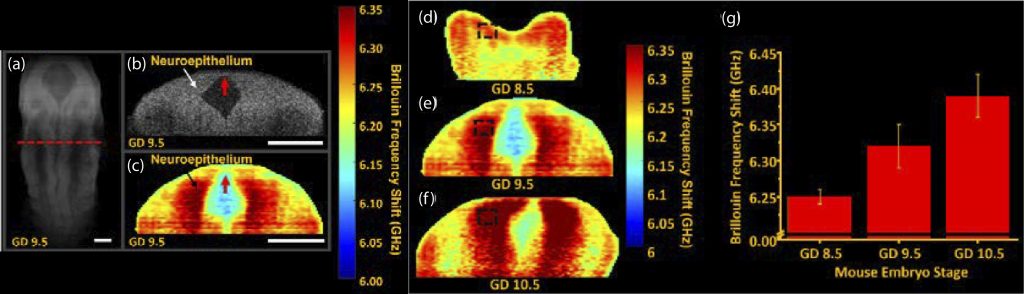
Imaging neural tube stiffness with a multimodal Brillouin microscopy and OCT system. (a) 3D OCT structural image of a murine embryo at gestational day (GD) 9.5. 2D (b) OCT and (c) Brillouin microscopy images of the selected plane shown as the red dashed line in (a). Neural tube stiffness in GD (d) 8.5, (e) 9.5, and (f) 10.5 embryos . (g) Quantification of the average neural tube stiffness as a function of development stage. Ambekar et al., Opt Lett, 2022.